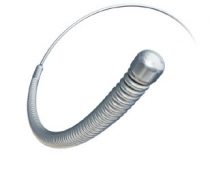
Boston Scientific is voluntarily recalling two core wires for its Rotablator Rotational Atherectomy System, according to an alert from the US Food and Drug Administration (FDA).
The manufacturer started recalling the guidewires back in October because of the possibility of cracking and separation that could lead to tamponade, MI and/or fragment migration. In fact, there have been three adverse events reported from these occurrences, including one death after a patient underwent an intervention to remove the damaged wire.
For more information about this device, please call us at 541-484-2434.

